Introduction
The intestinal microbiota plays a vital role in the health and development of infants, especially in the neonatal intensive care unit (NICU). Preterm infants, in particular, face unique challenges in establishing a healthy gut microbiota due to their premature birth and medical interventions. Probiotics, live microorganisms with potential health benefits, have been studied for their role in promoting a balanced gut microbiota and supporting overall health in preterm infants.
In this article, we explore the findings of a prospective study conducted at Orlando Health Winnie Palmer Hospital for Women & Babies, investigating the impact of a specific probiotic, Bifidobacterium infantis EVC001 (B. infantis EVC001), on preterm infants. The study aimed to evaluate the safety, tolerability, and effects of this probiotic on the gut microbiota and human milk oligosaccharide (HMO) utilization.
Study Design for B. infantis EVC001 Administration
This single-center, open-label, prospective study focused on the administration of B. infantis EVC001, a probiotic, to preterm infants in the NICU. The study protocol was approved by the Orlando Health Institutional Review Board. Unlike a randomized, placebo-controlled trial, this study was not blinded due to concerns from previous probiotic trials in NICU settings where infants in the placebo group inadvertently acquired the probiotic bacteria.
The target participants were preterm infants weighing less than 1,500 grams at birth or born before 33 weeks of gestational age, delivered either vaginally or by cesarean section. Detailed inclusion and exclusion criteria were established.
Materials and Methods
Data Collection
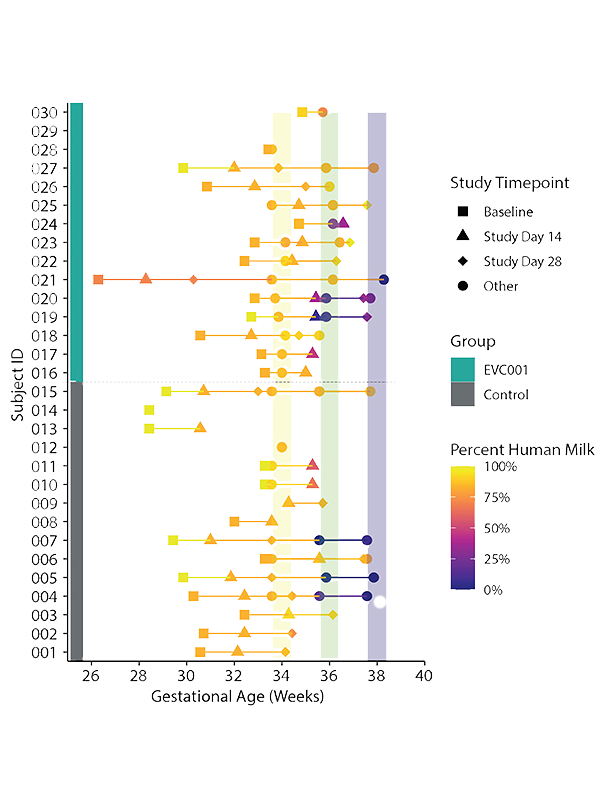
Baseline activities, including data collection and stool sample collection, took place before Day 10 of life. The study had two groups: a control group and an EVC001 group. The control group received no supplemental study product, while the EVC001 group received a daily feeding of 8.0 x 10^9 colony-forming units (CFU) of activated B. infantis EVC001 in medium-chain triglyceride (MCT) oil.
Medical events and diagnoses occurring before Study Day 0 were collected as Baseline Clinical Data. Information on nutritional intake, feeding mode, and concomitant medications was recorded weekly. Stool specimens were collected at six timepoints during the study.
Fecal DNA Extraction and Microbiota Studies
DNA was extracted from stool swab samples and quantified. Shotgun metagenomic sequencing and 16S ribosomal RNA (rRNA) gene sequencing were performed. Taxonomic profiling of the metagenomic samples was carried out using MetaPhlAn2, and functional metagenomics were profiled using HUMAnN2. Functional HMO utilization gene profiles were generated with HUMAnN2.
16S rRNA gene sequences were analyzed using QIIME2, and diversity analysis was performed to compare the microbiota composition between the groups. Absolute quantification of fecal B. infantis and HMOs was achieved through real-time PCR and high-performance liquid chromatography mass spectrometry (HPLC-MS), respectively.
Results of B. infantis EVC001 Supplementation
Recruitment, Demographics, and Baseline Clinical Data
- Thirty preterm infants were enrolled in the study.
- The average gestational age at enrollment was 30.3 weeks for the control group and 31.3 weeks for the EVC001 group.
- There were significant differences in race and ethnicity between the groups.
- Infants in the EVC001 group had significantly fewer days on total parenteral nutrition (TPN).
Safety and Tolerability
- Infants in the EVC001 group received daily feeds of the probiotic without significant adverse events.
- There were no significant differences in the number of adverse events between the EVC001 and control groups.
- Weight gain and other growth parameters were not significantly different between the groups.
- Stooling patterns differed significantly between control and EVC001 groups.
Microbiota Analysis Following B. infantis EVC001 Administration
- B. infantis was undetectable in all samples at baseline but significantly increased in the EVC001 group at Study Days 14 and 28.
- The Bifidobacteriaceae family significantly increased in the EVC001 group at both Day 14 and Day 28.
- Overall diversity differed significantly between the treatment groups, suggesting a strong effect of EVC001 supplementation.
- Binary Jaccard distances increased in the control group over time.
HMO Utilization
- Genes involved in HMO metabolism were abundant in the metagenomes of infants in the EVC001 group but absent in the control group.
- Absolute abundance measurements of HMOs in fecal samples showed a nearly 250-fold difference between the groups, with HMOs being much more abundant in the control group.
- The abundance of HMOs in fecal samples was negatively correlated with the absolute abundance of B. infantis.
Discussion
The study results indicate that supplementation with B. infantis EVC001 led to significant changes in the fecal microbiota composition and enhanced the metabolism of HMOs in preterm infants. Despite differences in HMO utilization, the study found no significant safety concerns associated with the use of EVC001.
This research provides valuable insights into the potential benefits of probiotic supplementation in preterm infants and underscores the importance of understanding the impact of probiotics on the gut microbiota and HMO utilization.
Further research is warranted to explore the long-term effects and clinical implications of these findings in preterm infant care.
In conclusion, this prospective study sheds light on the complex relationship between probiotics, gut microbiota, and HMO utilization in preterm infants and opens avenues for future investigations in neonatal care.


