Introduction
Mass spectrometry in metabolomics (LC-MS, GC-MS) is a powerful analytical approach that enables precise biomarker detection, metabolic pathway analysis, and advancements in personalized medicine and drug metabolism. Learn more about metabolomics services.
By studying metabolites—the small molecules involved in cellular processes—researchers can gain valuable insights into disease mechanisms, drug responses, and human health. This field has transformed our ability to detect diseases early, develop targeted treatments, and understand complex biological systems.
Mass spectrometry (MS) has emerged as the cornerstone technology in metabolomics research, offering unprecedented precision in detecting and measuring metabolites. Its ability to analyze thousands of molecules simultaneously has revolutionized our understanding of cellular processes and disease mechanisms.
Understanding Metabolomics and Its Role in Biomarker Discovery
Metabolomics, combined with high-resolution mass spectrometry (HRMS), LC-MS, and GC-MS, enables detailed analysis of metabolic pathways and biomarker discovery for disease diagnostics and drug development. What is metabolomics?
These include:
- amino acids
- proteins
- sugars
- carbohydrates
- lipids
- fatty acids
- nucleic acids
- hormones
- vitamins
- various signaling molecules
However, many more molecules can be found, including those related to drugs, environmental exposures, diet, etc.
Through the study of these compounds, researchers can understand crucial aspects of cellular function, including:
- energy production
- nutrient processing
- waste elimination
- cell signaling
This comprehensive analysis provides invaluable insights into how cells respond to environmental stressors and how diseases develop and progress.
The field has transformed modern medicine by enabling early disease detection, improving treatment development, and advancing personalized medicine approaches. Through metabolomic analysis, researchers can identify disease biomarkers before traditional symptoms appear, monitor treatment responses with unprecedented precision, and develop targeted therapeutic strategies based on individual metabolic profiles.
Mass Spectrometry Techniques in Metabolomics: LC-MS, GC-MS, and More
Mass spectrometry in metabolomics, particularly LC-MS and GC-MS, is an essential analytical technique for:
- Metabolic profiling
- Biomarker discovery
- Precision medicine
It works by measuring the mass of charged molecules (ions).
Chromatographic Separation in Mass Spectrometry
For complex biological samples, chromatographic separation often precedes mass spectrometry analysis. This preliminary step separates compounds based on their physical or chemical properties before they enter the mass spectrometer.
Two primary approaches are widely used for analyzing metabolic pathways and identifying biomarkers:

- LC-MS (Liquid Chromatography-Mass Spectrometry) – separates compounds in a liquid phase and works well for larger, less volatile molecules.
- GC-MS (Gas Chromatography-Mass Spectrometry) – separates volatile compounds in the gas phase.
This separation step greatly enhances the mass spectrometer’s ability to analyze complex mixtures by preventing too many compounds from entering the instrument simultaneously.
Ionization Process in Mass Spectrometry
After chromatographic separation, the process continues with ionization, where molecules are converted into charged particles.
Several ionization methods exist, each suited to different types of molecules and research needs:
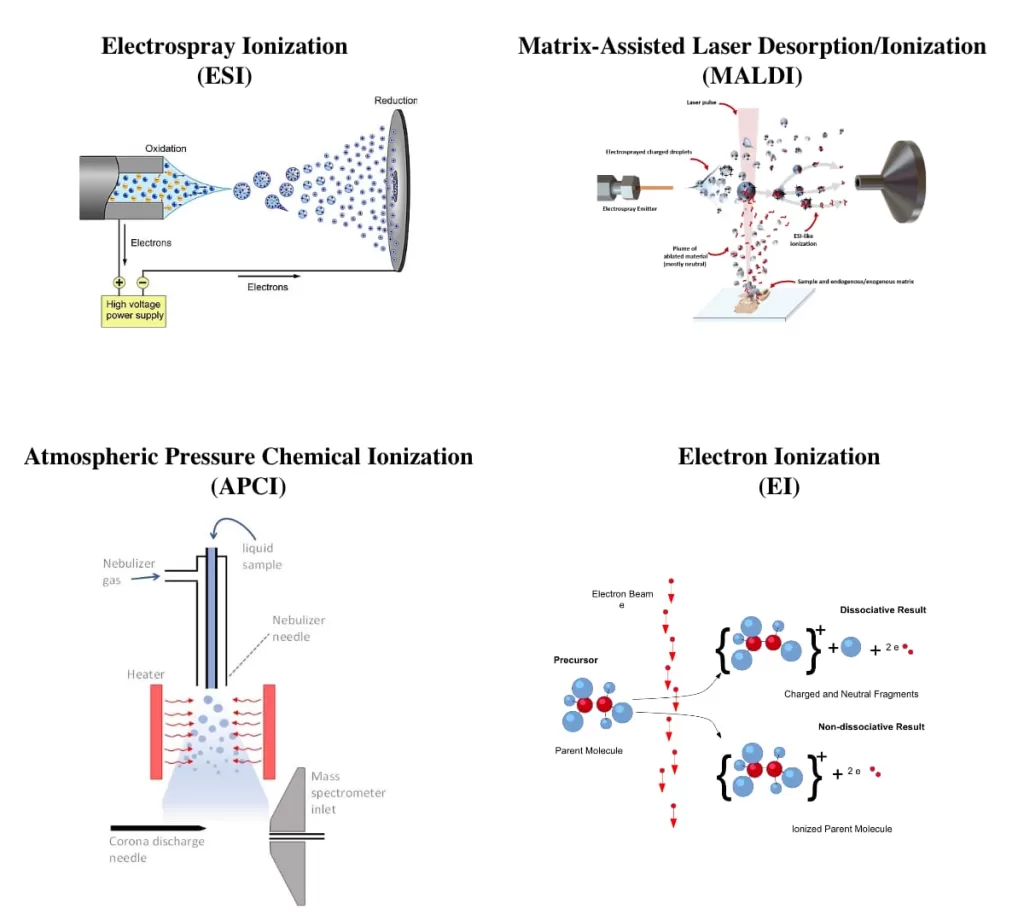
- Electrospray Ionization (ESI) – Excels at analyzing large, complex molecules.
- Matrix-Assisted Laser Desorption/Ionization (MALDI) – Proves ideal for tissue imaging.
- Atmospheric Pressure Chemical Ionization (APCI) – Works effectively for smaller molecules.
- Electron Ionization (EI) – Remains the standard method for volatile compounds.
Mass Analyzers in Mass Spectrometry
Once ionized, molecules pass through mass analyzers that separate them based on their mass-to-charge ratio.
Modern instruments employ various analyzer types, including:
- Quadrupoles
- Time-of-Flight (TOF) Systems
- Ion Traps
- Orbitraps
Each offers distinct advantages: quadrupoles provide excellent selectivity, time-of-flight analyzers deliver high resolution, and orbitraps achieve exceptional mass accuracy.
The most advanced systems, such as Fourier Transform Ion Cyclotron Resonance (FT-ICR) analyzers, can achieve ultimate resolution for complex mixture analysis.
Detection Systems
Detection systems then measure the separated ions, converting their presence into measurable signals. Common detectors include:
- electron multipliers
- microchannel plate detectors
- ion-to-photon detectors
These sophisticated components work together to generate detailed molecular profiles of biological samples.
Sample Preparation and Analysis
The quality of sample preparation in mass spectrometry is crucial for accurate metabolomics analysis. Techniques such as liquid-liquid extraction, solid-phase extraction, and supercritical fluid extraction are commonly used to isolate metabolites and enhance analytical sensitivity.
Effective sample collection requires immediate processing and appropriate storage conditions to preserve metabolite integrity. Samples must be rapidly frozen and protected from degradation factors such as:
- temperature fluctuations
- light exposure
- oxidation
Chemical stabilizers may be employed to maintain sample quality during storage and processing.
Advanced Sample Preparation Methods
Modern metabolomics research has driven the development of sophisticated sample preparation techniques. Microextraction methods now allow analysis of extremely small sample volumes, crucial for applications like single-cell metabolomics or precious clinical samples. Automated sample preparation systems have improved reproducibility and throughput while reducing human error.
Selective enrichment techniques help researchers focus on specific classes of metabolites. Affinity-based methods use specially designed materials to capture molecules of interest, while chemical derivatization can improve the detection of particular compound classes. These approaches have proven particularly valuable for studying low-abundance metabolites that might otherwise be masked by more prevalent compounds.
Quality control procedures have become increasingly standardized, with many laboratories adopting automated sample tracking systems and rigorous validation protocols. Internal standards, added at various stages of sample preparation, help monitor and correct for any variations in sample processing or instrument performance.
Sample preparation involves several critical steps to isolate and concentrate metabolites of interest. Extraction methods such as:
- liquid-liquid extraction
- solid-phase extraction
- supercritical fluid extraction
separate desired compounds from complex biological matrices. Following extraction, samples undergo clean-up procedures including filtration, centrifugation, and sometimes derivatization to improve analysis quality.
Data Analysis and Interpretation
The analysis of mass spectrometry data requires sophisticated processing tools and expertise. Raw data undergoes extensive signal processing, including:
- peak detection
- alignment
- baseline correction
- noise reduction
Mass calibration ensures accuracy, while feature detection identifies isotope patterns, adducts, and fragments. These processes transform raw instrumental output into meaningful molecular information.
Modern data analysis relies on specialized software platforms that combine statistical analysis with database searching. Tools like MetaboAnalyst, XCMS, and MZmine provide comprehensive analysis capabilities, while databases such as METLIN, the Human Metabolome Database, and LIPID MAPS offer extensive molecular reference information. These resources enable researchers to identify compounds and understand their biological significance.
Applications of Mass Spectrometry in Medical Diagnostics and Drug Development
Mass spectrometry for metabolomics, particularly LC-MS and GC-MS techniques, plays a crucial role in biomarker discovery, metabolic profiling, and precision medicine, enabling breakthroughs in medical diagnostics and drug development.
This technology plays a crucial role in studying cancer metabolomics, cardiovascular disease, and precision medicine, among many other.
In disease research, it enables detailed analysis of metabolic pathways, monitoring of disease progression, and identification of new biomarkers. This technology has proven particularly valuable in understanding complex conditions like cancer, diabetes, and neurodegenerative disorders.
Learn more about metabolomics in drug development
Disease-Specific Applications
Medical research has greatly benefited from mass spectrometry analysis. Researchers can now identify specific metabolic signatures that distinguish cancer cells from healthy cells, leading to improved diagnostic tools and treatment strategies. For example, mass spectrometry has revealed how cancer cells alter their glucose metabolism, known as the Warburg effect, providing new targets for therapeutic intervention. Recent studies have also shown how different cancer types exhibit unique metabolic profiles, enabling more precise diagnosis and treatment selection.
In neurodegenerative disease research, mass spectrometry helps track changes in brain metabolism that occur during disease progression. Researchers have identified specific metabolic markers for conditions like Alzheimer’s disease, potentially enabling earlier diagnosis and intervention. The technology can detect subtle changes in neurotransmitter levels and lipid metabolism, providing new insights into disease mechanisms and potential therapeutic approaches.
Cardiovascular disease research has also been transformed by mass spectrometry. The technology enables detailed analysis of lipid profiles and metabolic markers associated with heart disease, helping identify patients at risk before symptoms develop. Researchers can study how different dietary patterns and medications affect cardiovascular health at the molecular level, leading to more effective prevention strategies and treatments.
Therapeutic Development and Monitoring
Mass spectrometry plays a crucial role in drug development and monitoring. During the drug discovery phase, researchers use the technology to:
- Screen potential drug candidates for metabolic stability
- Study drug-protein interactions
- Identify unexpected metabolites that could cause side effects
- Monitor drug concentrations in different tissues
In clinical trials, mass spectrometry helps researchers understand how different patient populations metabolize drugs, enabling more precise dosing recommendations. The technology can track both the primary drug compound and its metabolites, providing a complete picture of drug processing in the body. This detailed understanding helps researchers optimize drug formulations and delivery methods, ultimately improving treatment outcomes.
In drug development, mass spectrometry helps identify therapeutic targets, study mechanisms of action, and assess drug toxicity. Researchers can track how drugs move through biological systems, understand their metabolic processing, and optimize dosing strategies. This detailed molecular insight has accelerated drug development and improved treatment outcomes.
Clinical applications extend to diagnostic testing, treatment monitoring, and personalized medicine. Hospitals and research centers use mass spectrometry to screen for inherited metabolic disorders, monitor treatment effectiveness, and adjust therapeutic approaches based on individual patient responses. This technology has become particularly important in oncology, where it helps guide treatment selection and monitor disease progression.
Current Challenges and Future Developments
Despite its power, mass spectrometry in metabolomics faces several challenges, including automation, AI-driven data analysis, and real-time metabolomics. However, advancements in portable mass spectrometers are making this technology more accessible.
Technical limitations include resolution constraints, sensitivity thresholds, and matrix effects that can complicate analysis. One of the most significant bottlenecks remains data processing and interpretation. Modern mass spectrometers can generate gigabytes of data in a single day, but extracting meaningful biological insights from this data continues to be challenging. Even with advanced software tools, the process of peak identification, alignment, and normalization requires significant time and expertise. Many laboratories struggle with the computational infrastructure needed to process and store these massive datasets effectively. Furthermore, the lack of standardized data processing workflows means that different laboratories may arrive at different conclusions from the same raw data.
Data analysis presents additional challenges, requiring sophisticated computational resources and expertise to handle large, complex datasets. The field particularly lacks robust automated solutions for metabolite identification—many compounds detected in biological samples remain unidentified or tentatively identified at best. This “dark matter” of metabolomics represents a significant challenge for researchers trying to understand complete metabolic pathways and their biological significance.
Technological Innovations
Recent technological advances are addressing many traditional limitations of mass spectrometry. High-resolution mass analyzers now offer unprecedented mass accuracy, enabling researchers to distinguish between molecules that differ by mere fractions of an atomic mass unit. This capability has proven crucial for identifying unknown metabolites and understanding complex biological pathways.
Ion mobility mass spectrometry represents another significant advancement. This technology adds an additional separation dimension based on molecular shape, helping researchers distinguish between molecules with identical masses but different structures. This capability has proven particularly valuable in protein analysis and the study of molecular conformations.
Miniaturization efforts are making mass spectrometry more accessible for point-of-care applications. Researchers are developing portable mass spectrometers that maintain high analytical performance while reducing size and cost. These developments could enable rapid, on-site analysis in clinical settings, environmental monitoring, and field research.
Computational Advances
Artificial intelligence and machine learning are revolutionizing mass spectrometry data analysis. New algorithms can:
- Automatically identify peaks and patterns in complex spectra
- Predict molecular structures from mass spectrometry data
- Detect subtle changes in metabolic profiles that might indicate disease
- Integrate mass spectrometry data with other analytical techniques
These computational tools are particularly important for handling the massive datasets generated by modern mass spectrometers. Deep learning approaches are improving the accuracy of metabolite identification and enabling more sophisticated pattern recognition in metabolomic data.
Cost remains a significant barrier to widespread adoption, as both equipment and maintenance require substantial investment. Additionally, the need for specialized technical expertise in operation and data analysis can limit accessibility. However, ongoing technological advances are addressing these challenges through improved automation, enhanced sensitivity, and more user-friendly interfaces.
Recent developments in single-cell analysis represent a particularly exciting frontier in mass spectrometry. This capability allows researchers to study cellular heterogeneity with unprecedented detail, providing crucial insights into cancer biology, stem cell research, and developmental processes. Similarly, advances in imaging mass spectrometry combine molecular analysis with spatial information, enabling detailed tissue studies and drug distribution mapping.
Conclusion
Mass spectrometry (mostly as LC-MS and GC-MS) remains at the forefront of metabolomics research, driving biomarker discovery, precision medicine, and disease diagnostics. With advancements in automation and AI-driven data analysis, mass spectrometry is becoming more accessible for medical diagnostics and drug development, while continuously evolving to offer sophisticated capabilities for analyzing complex biological systems. Explore our metabolomics services
As technology advances, its applications in research and clinical practice continue to expand. The integration of advanced technologies, improved data analysis methods, and novel applications across scientific disciplines promises to further enhance our understanding of biological systems at the molecular level.
A particularly exciting development is mass spectrometry’s potential expansion into consumer markets. As miniaturization advances and costs decrease, we may soon see mass spectrometry-based devices in healthcare clinics, pharmacies, and eventually even homes. These simplified, user-friendly instruments could enable rapid health monitoring, medication verification, or food safety testing. Several companies are already developing portable mass spectrometers that maintain adequate analytical performance while significantly reducing size and complexity. This democratization of mass spectrometry technology could revolutionize personal health monitoring and preventive medicine.
The future of mass spectrometry in metabolomics lies in continuing technological refinement, enhanced accessibility, and broader application across research and clinical domains. These advances will likely lead to new discoveries in disease mechanisms, improved therapeutic strategies, and more personalized approaches to medical treatment. As the field continues to develop, mass spectrometry will remain essential to advancing our understanding of human health and disease, while potentially becoming an integral part of everyday healthcare and consumer wellness monitoring.
Frequently Asked Questions
What distinguishes mass spectrometry from other analytical techniques?
Mass spectrometry offers unparalleled molecular specificity and sensitivity compared to traditional analytical methods. While techniques like spectrophotometry or chromatography can measure general chemical properties, mass spectrometry can identify and quantify specific molecules within complex mixtures. This capability makes it particularly valuable for analyzing biological samples containing thousands of different compounds.
How does LC-MS and GC-MS mass spectrometry improve biomarker discovery, metabolic profiling, and disease diagnostics in precision medicine?
Mass spectrometry enables the detection of disease-specific molecular markers long before traditional symptoms appear. For example, in newborn screening programs, mass spectrometry can identify inherited metabolic disorders from a single drop of blood, allowing for early intervention and treatment. In cancer diagnosis, it can detect subtle changes in cellular metabolism that indicate the presence of tumors.
Why is sample preparation so crucial in mass spectrometry?
Sample preparation directly affects the quality and reliability of mass spectrometry results. Proper preparation removes interfering substances, concentrates molecules of interest, and ensures sample stability. Poor preparation can lead to false results or missed detections, particularly when analyzing complex biological samples like blood or tissue.
How is mass spectrometry advancing personalized medicine?
Mass spectrometry helps identify individual differences in how patients metabolize drugs and respond to treatments. This information allows healthcare providers to select optimal medications and dosages based on a patient’s unique molecular profile. The technology also helps monitor treatment effectiveness by tracking changes in metabolite levels over time.
What are the main challenges in implementing mass spectrometry in clinical settings?
While mass spectrometry offers powerful analytical capabilities, its implementation faces several challenges:
- High initial equipment and maintenance costs
- Need for specialized technical expertise
- Complex data analysis requirements
- Time required for sample preparation and analysis However, ongoing technological advances are making the technology more accessible and easier to use in clinical environments.
How does mass spectrometry support drug development?
Mass spectrometry plays multiple roles in drug development, from initial discovery to clinical trials. It helps:
- Identify potential drug targets
- Study how drugs are metabolized
- Monitor drug levels in clinical trials
- Detect potential side effects This comprehensive analysis capability has become essential for modern pharmaceutical research.
More answers to frequent questions about Mass Spetrometry can be found here: Analytical Techniques in Metabolomics: Mass Spectrometry, NMR, and Emerging Technologies.
